November 16, 2016 Essure FDA Update
On November 16, 2016 the FDA released a statement requiring the addition of a boxed warning and a Patient Decision Checklist intended to support patient understanding of benefits and risks associated with Essure. Here is a suggested example of a new Essure boxed warning from the FDA:

The boxed warning aka “black-box” is the strongest warning the FDA requires. It is an indication that the FDA has concluded there is a serious risk of significant or life-threatening injuries when using the device or medication. Those considering permanent birth control with Essure should strongly consider the new warnings from the FDA before choosing this form of birth control. What legal remedies would be available to women who have the device implanted after this warning takes effect is uncertain. If you have already been injured by the Essure device, this boxed warning could dramatically impact the time frame in which your lawsuit should be filed. Contact us today at 1.800.285.4878 or by filling the form out on the side.

800-285-HURT (4878)Available 24/7 | 356 Days | se habla español
August 2, 2016 Essure Litigation Update
Judge Winifred Y. Smith of the Superior Court in Alameda County has cleared the path for nearly a dozen Essure lawsuits to proceed in California. Bayer sought to have the cases thrown out on three separate grounds, but on each of them Judge Smith sided with the plaintiffs. The rulings filed in August mean that Bayer could still be held liable for the harm alleged in current lawsuits and also pave the road for future lawsuits over Essure.
March 23, 2016 Essure Litigation Update
U.S. District Judge John Padova ruled that Plaintiffs could continue their product liability suits against Bayer. Judge Padova dismissed most counts of the Plaintiffs’ lawsuits against Bayer but he gave them the option to re-plead those counts. In addition to being allowed to re-plead the dismissed claims, Plaintiffs claims of negligent misrepresentation and negligent failure-to-warn claims against Bayer survived as originally plead.
The Pennsylvania ruling is in direct contrast to the ruling in the California case just a few weeks ago where claims against Bayer were dismissed under the legal theory of preemption due to the type of FDA approval originally received for the device.

800-285-HURT (4878)Available 24/7 | 356 Days | se habla español
March 4, 2016 Essure Litigation Update
In California some of the claims were dismissed with prejudice and some were dismissed without prejudice. No word on whether or not those cases will be refiled. The litigation in Pennsylvania is ongoing as we wait for the judge to make a final ruling.
February 29, 2016 Essure FDA Update
On February 29, 2016 the FDA took several actions related to the Essure permanent birth control device. These actions include:
- The Agency intends to require a mandatory box warning on the product explaining the adverse events that have been associated with these devices, including their insertion and/or removal procedures.
- The draft guidance also includes proposed language for the “patient decision checklist,” for doctors to discuss with patients to better communicate risks and help to ensure an informed decision-making process.
- The FDA has also ordered Bayer, the company that manufactures Essure, to conduct a new postmarket surveillance study designed to provide important information about the risks of the device in a real-world environment.
- The study will also evaluate how much these complications affect a patient’s quality of life.
To learn how the updated labeling affects Essure lawsuits, contact us today at 1.800.285.4878.
January 15, 2016 Essure Litigation Update
Because the Essure device went through the FDA’s premarket approval process, Bayer is currently shielded from product liability lawsuits involving the implant. However there is currently an Essure case pending in the U.S. District Court, Eastern District of Pennsylvania, seeking to remove that protection (Case No. 2:2015cv00384). It is alleged that the results of the clinical trials that provided Conceptus & Bayer with legal immunity were falsified to gain FDA approval to sell the device. Loncar Lyon Jenkins is closely monitoring the litigation a decision is expected shortly.
Should the premarket approval become invalidated, Loncar Lyon Jenkins will be free to pursue litigation in order for victims of Essure side effects to receive due compensation. Contact us today so that we may act quickly in the event of a favorable ruling.
Loncar Lyon Jenkins is now investigating Essure birth control injuries for litigation
The FDA has received numerous reports of pain or health complications as a result of the Essure IUD birth control. From November 4, 2002, Essure’s approval date, through May 31, 2015, the FDA received 5093 medical device reports related to problems with Essure. The majority of complaints received since 2013 have been voluntary reports, mostly from women who received Essure implants. The most frequently reported side-effects were:
- Pain/abdominal pain (3353)
- Heavier menses/menstrual irregularities (1408)
- Headache (1383)
- Fatigue (966)
- Weight fluctuations (936).
Most of the reports received listed multiple patient problems in each report.
The most frequent device problems reported were:
- Patient device incompatibility (941) (e.g., nickel allergy)
- Migration of the device or device component (482)
- Device operating differently than expected (301)
- Device breakage (259)
- Malposition of the device (133)
- Unintended pregnancy with birth defects
In addition, many women injured by Essure reportedly needed to undergo hysterectomies in order to correct the problems caused by the device. Today there are centers which specialize in safely removing the Essure device without having to undergo a hysterectomy. Instead a microsurgery can be performed in order to safely remove all three components of the device.
What is Essure?
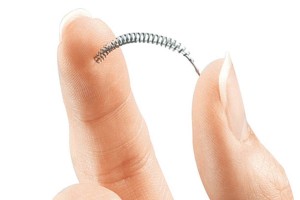
Originally manufactured by Conceptus, Inc., Essure is an IUD (intrauterine device) form of birth control. It is a four-centimeter, micro-insert. Comprised of an expanding coil made of stainless steel, a nickel Titanium expanding outer coil, and polyethylene fibers. The micro-inserts are placed directly into the Fallopian tubes where the coils expand upon release. Due to inflammation brought on by the polyethylene fibers, scar tissue builds up obstructing the tubes and permanently preventing fertilization. Conceptus received FDA premarket approval (PMA) for Essure November 4, 2002. It was touted as a breakthrough alternative to surgical sterilization. In 2013 Conceptus was acquired by Bayer for approximately $1.1 billion.
If you or someone you love were among those injured as a result of an Essure implant, please contact us immediately. Loncar Lyon Jenkins is investigating cases in all 50 states. We will prove you with a free, confidential case evaluation using the form on the side or by calling toll free at 800.285.4878. Essure lawsuits are being evaluated on a confidential, contingency fee basis, you pay nothing unless a recovery is obtained.