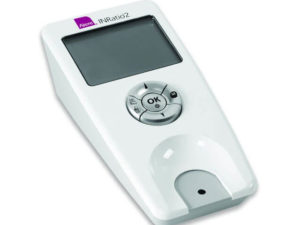
Loncar Lyon Jenkins is now filing INRatio recall lawsuits in order to ensure victims of INRatio device failure and bleeding events receive due compensation
The Alere INRatio and INRatio 2PT/INR Monitor System and INRatio Test Strips measure how quickly a patient’s blood clots when taking Warfarin, an anticoagulant (blood thinner) . It uses The International Normalized Ratio (INR) test in order to compare the results of the patients’ Prothrombin Time (how quickly your blood clots). Trained patients and healthcare professionals use these Alere INRatio and INRatio 2PT/INR Monitor Systems and INRatio Test Strips to monitor the anti-coagulation levels in the bloodstream. Defects in these products have been shown to cause inconsistent and incorrect INR results when compared to laboratory INR methods. Defective readings due to the faulty devices have put misinformed patients in potentially injurious and fatal situations. The devices assure patients that they are maintaing safe INR levels, when they may in fact be in need of medical assistance. Failure to seek medical assistance at unsafe INR levels when taking Warfarin can lead to bleeding events and a variety of health complications. Known problems include:
- Intestinal bleeding
- Thrombosis (blood clots)
- Decreased hemoglobin
- Cerebral hemorrhaging
- Hematoma (semisolid mass in the blood)
- Peripheral edema (swelling of the lower limbs)
- Dyspnea (difficulty breathing)
- Stroke
- Mycordial Infarction (heart attack)
- Hospitalization for excessive bleeding
If you or someone you know has experienced these health complications as a result of INRatio, you may be eligible to file an INRatio recall lawsuit. Contact Loncar Lyon Jenkins immediately for a free, confidential case evaluation using the form or by calling toll free at 800.285.4878. INRatio lawsuits are being evaluated on a contingency fee basis, meaning nothing is owed unless a recovery is obtained.

800-285-HURT (4878)Available 24/7 | 356 Days | se habla español
Alere INRatio Recalls
Alere Inc. recalled INRatio2 PT/INR Professional Test Strips on April 16, 2014 due to instances of patients recording a beneficial INR when using the test strips, but a significantly higher INR on tests performed by a central laboratory. Manufactured dates for the affected test strips range from August 22, 2013 through April 2, 2014 with distribution from August 26, 2013 through April 2, 2014.
Alere issued a second recall statement on December 5, 2014 involving the Alere INRatio and INRatio2 PT/INR Monitor System. The release states that the systems should not be used on patients with any of the following conditions:
- Anemia of any type with hematocrit less than 30%
- Any conditions associated with elevated fibrinogen levels including:
- Acute inflammatory conditions (examples may include acute viral or bacterial infections such as pneumonia or influenza)
- Chronic inflammatory conditions (examples may include rheumatoid arthritis, Crohn’s disease, ulcerative colitis, infectious liver diseases such as hepatitis, or inflammatory kidney diseases such as diabetic nephropathy and glomerulonephritis)
- Severe infection (e.g., sepsis)
- Chronically elevated fibrinogen for any reason
- Hospitalized or advanced stage cancer or end stage renal disease patients requiring hemodialysis
- Any bleeding or unusual bruising, clinically observed or reported by the patient
This recall involves the Alere INRatio Monitor, INRatio 2 Monitor, and INRatio Test Strips manufactured and distributed from April 1, 2008 to December 4, 2014. The FDA advises that patients with any of the conditions listed above should immediately be transitioned to a laboratory INR method for monitoring their INR levels.
Both recalls of the Alere INRatio monitoring system and test strips were updated to a “Class I” recall on January 9, 2015. The FDA defines a Class I recall as “a situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.” If you or someone you know has experienced serious adverse health consequences or death as a result of INRatio, you may be eligible to file an INRatio recall lawsuit. Contact Loncar Lyon Jenkins immediately for a free, confidential case evaluation using the form on the side or by calling toll free at 800.285.4878. INRatio lawsuits are being evaluated on a contingency fee basis, meaning nothing is owed unless a recovery is obtained.